Background: Treatment of chronic lymphocytic leukemia (CLL) has been transformed with targeted therapies including inhibitors of Bruton's tyrosine kinase (BTKi). Currently three covalent BTK inhibitors (cBTKi) are approved for CLL, but most patients eventually relapse, commonly through acquisition of the C481S BTK mutation (Woyach et al. 2014). Pirtobrutinib and nemtabrutinib are non-covalent BTK inhibitors (ncBTKi) developed to target and inhibit C481S mutant BTK. However novel secondary site mutations in BTK, namely T474I and L528W, have been found to confer resistance to both cBTKi and ncBTKi (Wang et al. 2022). Regardless of these mutations, BCR signaling remains intact, suggesting that inhibition of BTK maintains its therapeutic importance. LP-168 is a novel ultra-selective 4 th generation BTKi with an active warhead capable of covalent interaction with WT BTK or non-covalent binding when a BTK C481 mutation is present.
Methods: To determine the target selectivity of LP-168 we screened the compound against 468 kinases in the scanMAX Kinase Assay. For validation of covalent binding, dialysis against WT BTK was performed. Primary CLL B cells were isolated by negative selection and treated with LP-168 for all experiments. BCR signaling alterations were assessed via immunoblot to observe changes in target protein phosphorylation. Changes in CLL migration towards CXCL12 or CXCL13 were interrogated by transwell migration. Cytotoxicity towards primary CLL cells was measured via Annexin V/PI flow cytometry with and without HS-5 stromal cell support. Experiments using TMD8 cells were performed following CRISPR modification to insert WT, C481S, or T474I BTK. In vivo survival studies were performed using both the Eμ-TCL1 and Eμ-MTCP1 mouse engraftment models.
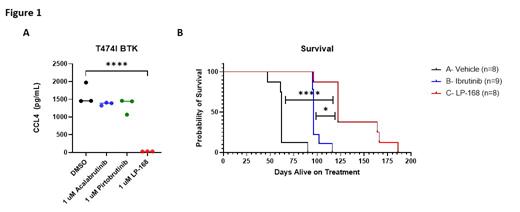
Results: LP-168 demonstrated selectivity towards BTK with roughly 700-fold selectivity for BTK vs its next off-target. In an enzymatic assay, LP-168 displayed nanomolar potency towards both WT BTK (IC 50=0.11 nM) and C481S BTK (IC 50=1.0 nM). Following 2-hour drugging, LP-168 inhibited BCR signaling in primary CLL B cells at increasing concentrations, demonstrated by reduced phosphorylation of BTK (92%, p<0.0001, n=8) and its immediate downstream target PLCγ2 (41%, p=0.0013, n=8). Migration capacity of primary CLL cells towards CXCL12 and CXCL14 was also found to decrease after 2-hours of treatment with LP-168 (52%, p<0.001, n=10 and 51%, p<0.001, n=10, respectively). After 48 hours of exposure, LP-168 was able to induce cytotoxicity of primary CLL cells alone and in co-culture with HS-5 stromal cells in a dose-dependent manner, with similar or improved efficacy to other BTKi. LP-168 decreased CLL cell production of CCL3 and CCL4 chemokines (CCL3: 93%, p<0.001, n=8; CCL4: 93%, p<0.001, n=8). LP-168 also demonstrated modest cytotoxicity towards TMD8 cells harboring either C481S BTK (24%, p=0.0008 , n=3) or T474I BTK (13%, p=0.0690, n=3), as well as decreased production of CCL3 (C481S: 95%, p<0.0001, n=3; T474I: 99%, p<0.0001, n=3) and CCL4 (C481S: 97%, p<0.0001, n=3; T474I: 98%, p<0.0001, n=3) (Figure 1A). Further, we observed inhibition of downstream BCR signaling in primary CLL B cells harboring C481S via immunoblotting and cytokine production. Finally, to evaluate the in vivo efficacy of LP-168 we tested this compound in both the Eµ-TCL1 and Eµ-MTCP1 models. We treated mice daily via oral gavage and found that 50 mg/kg of LP-168 significantly improved survival in the Eµ-TCL1 model when compared to vehicle (median 51 vs 44 days; p=0.0018) or ibrutinib at 50 mg/kg (median 51 vs 45 days; p=0.0098) and the Eµ-MTCP1 model when compared with vehicle (median 122 vs 62 days; p<0.0001) or ibrutinib at 50 mg/kg (median 122 vs 96 days; p=0.0162) (Figure 1B).
Conclusions: Collectively, our data demonstrate that LP-168 is a potent and selective inhibitor of BTK with activity against CLL, even in the presence of mutations that mediate resistance to cBTKi and ncBTKi. These data support the continued preclinical and clinical investigation of LP-168, which is currently being studied in the phase 1 setting of CLL (NCT04775745) and NHL (NCT04993690).
Disclosures
Rogers:AstraZeneca: Consultancy; Beigene: Consultancy; Loxo@Lilly: Consultancy; Novartis: Research Funding; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy; AbbVie: Consultancy, Research Funding. Kittai:Eli Lilly: Consultancy; Janssen: Consultancy; Abbive: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; KITE: Consultancy; BMS: Consultancy. Bhat:Aptitude Health: Honoraria; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding. Byrd:Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Kay:Beigene: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmcyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; boehringer ingelheim: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Vincerx: Research Funding; Genentech: Research Funding; Bristol Meyer Squib / Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Behring: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chen:Newave Pharmaceutical Inc: Current Employment, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Newave Pharmaceutical Inc. Tan:Guangzhou Lupeng Pharmaceutical Co: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Newave Pharmaceutical Inc. Anthony:Newave Pharmaceutical Inc: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Exact Sciences: Consultancy; Halia Therapeutics: Consultancy; Sumitomo Dainippon Pharma oncology: Patents & Royalties. Chen:Newave Pharmaceutical Inc.: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding.